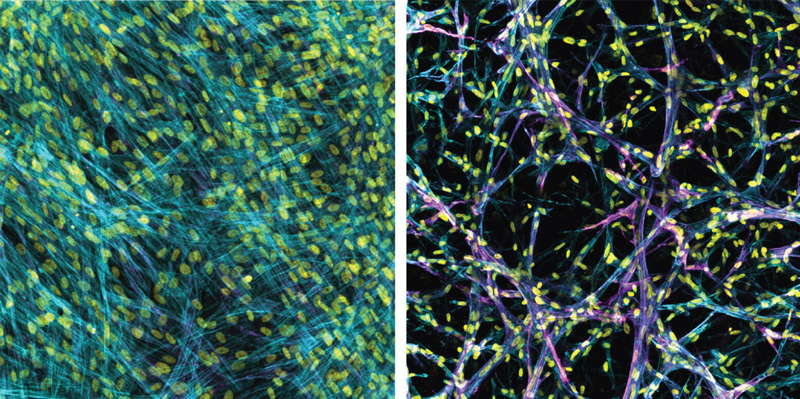
Researchers at the University of Michigan in Ann Arbor have created 3-D bioengineered lung tissue that shows how pulmonary fibrosis, a deadly lung disease, progresses.
Prior to the model, researchers only had access to observations made from Petri dishes, which are flat.
The causes of pulmonary fibrosis are not fully understood, but the condition is marked by scar tissue that forms inside the lungs. The scar tissue stiffens the walls of the lungs’ air sacs, called alveoli, or can completely fill them. Both scenarios make it difficult to breath and decreases the amount of oxygen entering the blood stream. The condition is often irreversible, eventually causing lung failure and death.
Researchers have found some drugs that relieve symptoms or slow the progression of the disease, but they haven’t been able to replicate the results in 2-D lab models that are currently available, making it impossible to understand why or how certain drugs work and making it difficult to predict which compounds will make a difference as they search for better treatments.
The 3-D tissue engineered model of fibrotic lung tissue shows that the drugs work.
Before the drug testing, the scientists performed studies to understand how tissue stiffness drives the appearance of myofibroblasts, or cells that correlate with the progression of scarring.
“Even in cells from the same patient, we saw different outcomes,” says Daniel Matera, a doctoral candidate and research team member. “When we introduced stiffness into the 2-D testing environment, it activated myofibroblasts, essentially creating scar tissue. When we introduced that same kind of stiffness into our 3-D testing environment, it prevented or slowed the activation of myofibroblasts, stopping or slowing the creation of scar tissue.”
Research on the 2-D scale showed that high lung stiffness was what clinicians needed to target. The new U-M research, however, indicates that targeting stiffness alone may not hinder disease progression in patients, even if it works in a Petri dish.
Brendon Baker, assistant professor in the U-M Department of Biomedical Engineering, and his team took a tissue engineering approach. They reconstructed 3-D lung interstitium, or connective tissue, the home of fibroblasts and location where fibrosis begins. Their goal was to understand how mechanical cues from lung tissue affect fibroblast behavior and disease progression.
“Recreating the 3-D fibrous structure of the lung interstitium allowed us to confirm effective drugs that wouldn’t be identified as hits in traditional screening settings,” Baker says.
The fibroblast is a cell found in the lung interstitium that is crucial to healing but can also drive disease progression. When activated after an injury or when a disease is present, they become myofibroblasts. When regulated, they help with wound healing, but when misregulated, they can drive chronic disease and symptoms such as the stiffening of lung tissue in pulmonary fibrosis.
“Our lung tissue model looks and behaves similarly to what we have observed when imaging real lung tissue,” Baker said. “Patient cells within our model can actively stiffen, degrade, or remodel their own environment just like they do in disease.”
The study was published in Science Advances and was funded in part by the National Institutes of Health. It is available here.











